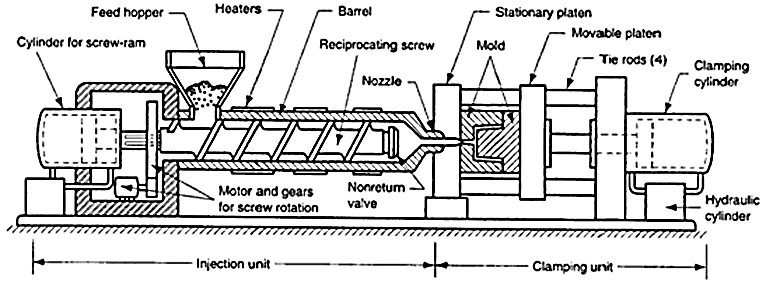
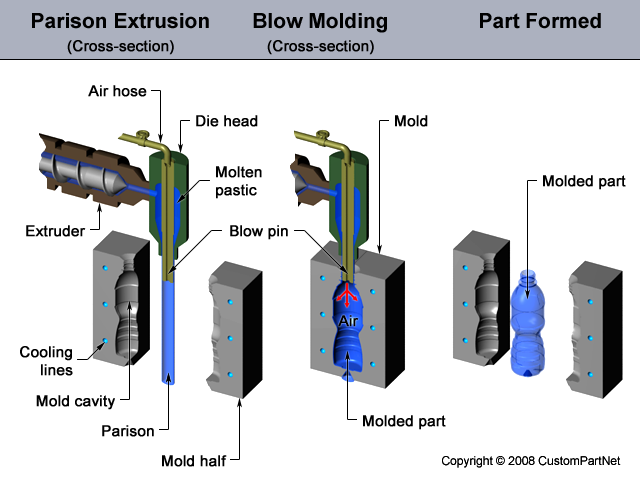
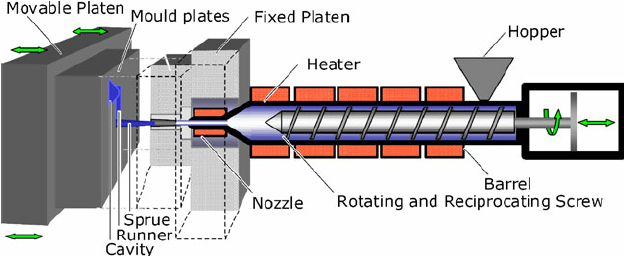
The conversion of raw polymers into finished products involves a series of polymer manufacturing processes. The first step consists of mixing additives into the polymer to achieve the required modification to the properties of the raw polymer.
The second stage is to create the desired shape. Inherent in the forming stage is the requirement to set or maintain that shape.
Forming can be conveniently divided into two-dimensional forming, where products have a relatively simple geometry, and also three-dimensional forming with complex geometry.
In most manufacturing there will be a number of finishing steps. The advantage of polymer processing over manufacturing with more traditional materials is that there are opportunities for cost savings through minimising finishing processes.
Manufacture of a particular product may require more than one forming process.
A crucial feature of most polymer processes is the preparation of the polymeric material in a appropriately softened state to suit the forming stage. Usually the softened state is achieved by heating the polymer.

Setting the shape is achieved by either cooling or carrying out a chemical process (crosslinking) to achieve the necessary dimensional stability.
Polymer manufacturing processes can be divided into continuous processes and batch processes. In continuous process where raw material is fed in continuously and the product flow appears continuously e.g. extrusion, there is more efficient use of energy and it is easier to maintain a consistent quality.
For batch or cyclic processes, such as moulding processes, there is a higher probability of batch-to-batch variation and lower efficiency due to unproductive parts of the cycle (down time).
Polymer manufacturing processes – The common features of polymer processing are:
Mass transfer
Energy transfer (mainly heat energy)
Flow and deformation (rheology)
Heat Transfer
Polymeric materials are characterised by high specific heat and also low thermal conductivity. Therefore, this makes them unsuitable for heating by conduction in thick sections. Consequently, the best form of feed is as finely divided granules or powders.
In conduction heating, the poor thermal conductivity results in undesirable temperature gradients and shear (frictional) heating is faster and also provides more uniform temperatures.
The energy required to raise unit mass of polymer from room temperature to processing temperature is defined as the enthalpy. Semi-crystalline thermoplastics have enthalpies almost double that of their amorphous counterparts.
Because of their susceptibility to thermal decomposition it is advisable to heat polymers up quickly, form quickly and cool down as soon as possible and avoid long residence times at elevated temperatures.

Rheology
In the melt state thermoplastics show varying resistance (viscosity) to applied flow stress.
Viscosity (resistance to flow) :
decreases with increasing temperature
increases with increasing pressure
decreases with increasing shear strain rate (shear thinning, pseudoplastic)
increases with increasing molecular size (MW)
decreases with increasing lubricant content
increases with increasing filler content
Polymer dispersions (latex, plastisols) can exhibit both shear thinning (pseudolplasticity) and shear thickening (dilatancy).
Because of their high viscosities, thermoplastic melts rarely show turbulent flow. In most situations it can be assumed that flow is laminar.
In an isothermal channel, such as an extrusion die where the wall is at the same temperature as the melt, the flow front will be parabolic. The highest velocity will be at the centre of the channel and reducing to zero at the wall.
In injection moulding, where a hot melt flows into cooler channels, a “frozen” skin layer is established at the wall with the melt flowing inside the skin in an unfolding melt front (“fountain flow”).